Background:
Thrombotic microangiopathy (TMA) associated with hematopoietic stem cell transplantation (HSCT-TMA) is a serious post-transplant complication. HSCT-TMA is difficult to diagnose due to overlapping symptoms with other conditions and a lack of universally adopted diagnostic criteria.
Objectives:
This study aimed to assess the incidence and diagnosis of HSCT-TMA in real-world practice across the US, including possible missed diagnoses, alongside clinical outcomes and associated burden of HSCT-TMA.
Methodology:
This retrospective, observational study investigated the incidence of HSCT-TMA from July 2009-August 2020 using data from the TriNetX US Electronic Medical Record (EMR) database. Patients who underwent autologous or allogenic HSCT procedures and had conditioning regimens recorded were stratified into three cohorts: confirmed TMA, suspected TMA, and non-TMA patients. Confirmed TMA was defined as any individual with at least one recorded hemolytic uremic syndrome (HUS)/TMA diagnosis code within 12 months of HSCT. Suspected TMA included individuals without a recorded HUS/TMA ICD code, but who met the modified published criteria (Cho BS, et al. Transplantation 2010;90:918-26; Jodele S, et al. Transfus Apher Sci 2016;54:181-90) within 12 months of HSCT; published criteria were adapted to fit data available in the TriNetX database. Non-TMA patients did not meet criteria to be included in either of the previous two cohorts. Adult and pediatric patients were analyzed separately. Demographic and clinical characteristics at baseline, clinical outcomes, and all-cause unadjusted healthcare resource utilization within 12 months of HSCT, were assessed. The baseline period was defined as the six months before the index date (inclusive), unless specified; index date was defined as the date a patient had a HSCT procedure code recorded. Statistical comparisons, where conducted, were made against the non-TMA cohort using the Fisher's exact test for categorical variables, the student's t test for means, and the Mann-Whitney U test for medians. Significance was set at the level of p<0.05.
Results:
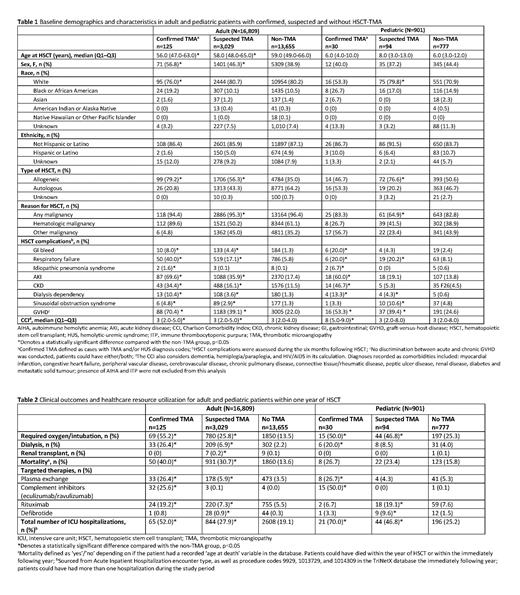
In total, 16,809 adult and 901 pediatric patients had an HSCT procedure code and a recorded conditioning regimen. The confirmed TMA group comprised 125 (0.7%) adult and 30 (3.3%) pediatric patients, the suspected TMA group included 3,029 (18.0%) adult and 94 (10.4%) pediatric patients, and the non-TMA group included 13,655 (81.2%) adult and 777 (86.2%) pediatric patients. Baseline demographics were similar across all groups. Mortality within the first year of HSCT was significantly higher in adult patients with confirmed TMA (40.0%) and suspected TMA (30.7%) compared with patients without TMA (13.6%). Mortality was numerically highest in pediatric patients with confirmed TMA (26.7%) followed by suspected TMA (23.4%), and lowest in non-TMA patients (15.8%). Comorbidity impact (based on the Charlson Comorbidity Index) was significantly higher, and HSCT complications significantly more common, in confirmed and suspected TMA patients (Table 1). Clinical outcomes in the first year after HSCT were significantly worse in confirmed and suspected TMA patients than in non-TMA patients (Table 2). All patients with confirmed and suspected TMA had a significantly higher frequency of oxygen/intubation compared with non-TMA patients. Dialysis rates were significantly higher in all patients with confirmed TMA (adults, 26.4%; pediatrics, 20.0%) and adult patients with suspected TMA (6.9%), compared with patients with no TMA (adults, 2.2%; pediatrics, 4.0%); pediatric patients with suspected TMA (8.5%) had numerically higher dialysis rates compared with those without TMA (4.0%). Patients with confirmed and suspected TMA had significantly greater use of targeted therapies and a higher number of total ICU visits, compared with non-TMA patients (Table 2).
Conclusions:
This study showed that HSCT-TMA is associated with a high mortality and morbidity burden. Furthermore, these results suggest that HSCT-TMA is underdiagnosed in the real-world setting, as patients with suspected TMA had worse outcomes and a higher burden of disease than patients without TMA following HSCT. This study highlights the need for timely diagnosis of this severe complication, as well as novel approaches to treat and manage this vulnerable population.
Disclosures
Wang:Alexion, AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Rava:Alexion, AstraZeneca: Research Funding; Genesis Research: Current Employment. Smuzynski:Genesis Research: Current Employment; Alexion, AstraZeneca Rare Disease: Research Funding. Shah:Genesis Research: Current Employment. Thanataveerat:Genesis Research: Current Employment. Al Dakkak:Alexion, AstraZeneca: Current Employment, Current equity holder in publicly-traded company. Ong:AstraZeneca: Other: Shareholder; Alexion, AstraZeneca Rare Disease: Current Employment. Dvorak:Allovir: Consultancy; Jazz Pharmaceuticals: Consultancy; Alexion, AstraZeneca Rare Disease: Consultancy. Ho:Orca Bio: Consultancy; Omeros: Consultancy; Allovir: Consultancy; Alexion, AstraZeneca Rare Disease: Consultancy; CareDx: Research Funding; Jazz Pharmaceuticals: Research Funding; Omeros: Research Funding; Alexion, AstraZeneca Rare Disease: Speakers Bureau.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal